Abstract
Background: Systemic light chain (AL) amyloidosis is a clonal plasma cell dyscrasia in which misfolded monoclonal light chains form insoluble extracellular fibrillar deposits with resultant organ injury. Renal and/or cardiac involvement is common. Clinical staging is based on cardiac involvement, with median survival of < 1 yr in advanced stage AL (Dispenzieri A, et al. J Clin Oncol 2004; Kumar S, et al. J Clin Oncol 2012). Therapy for AL is generally derived from that for multiple myeloma (MM). Combinations of immunomodulatory drugs (IMiDs®) and proteasome inhibitors are standard frontline MM therapy, but there is little experience with such regimens in AL. This trial evaluated pomalidomide (POM), bortezomib (BTZ) and dexamethasone (DEX) as frontline AL therapy.
Methods: Key eligibility: 1) AL with organ involvement; patients (pts) with light chain deposition disease (LCDD) also potentially eligible; 2) ≤ 1 cycle of prior systemic therapy for AL, excluding high dose melphalan; 3) Measurable disease, defined as a difference between the involved (amyloidogenic) and uninvolved free light chains of ≥ 5 mg/dL; 4) 18+ yrs of age; 5) ECOG performance status 0-2; 6) Serum creatinine ≤ 2.5 mg/dL; 7) serum NT-proBNP < 8500 pg/dL; 8) No myeloma, POEMS syndrome, or invasive cancer. Each cycle was 28 days, with POM administered PO on days 1-21, BTZ SQ on days 1,8, and 15, and DEX PO on days 1,8,15, and 22. The trial used a "3+3" design, with dose limiting toxicity (DLT) determined during cycles 1 and 2. Cohort 1: POM 2 mg/BTZ 1.0 mg/m2/DEX 40 mg. Cohort 2: POM 3 mg/BTZ 1.0 mg/m2/DEX 40 mg. Cohort 3: POM 4 mg/BTZ 1.0 mg/m2/DEX 40 mg. Cohort 4: POM 4 mg/BTZ 1.3 mg/m2/DEX 40 mg. Cohort 2b was added in an amendment: POM 3 mg/BTZ 1.3 mg/m2/DEX 20 mg. Acyclovir and aspirin (or anticoagulation) were required. DEX starting dose reduction to 20 mg was required for pts with cardiac AL, edema, or age > 70 yrs. Treatment continued until disease progression, unacceptable toxicity, withdrawal of consent, or physician discretion. The 1° endpoint was determination of maximally tolerated dosing (MTD) for POM/BTZ/DEX. Key 2° endpoints included hematologic and organ response rates, and overall survival (OS). Hematologic response (HR) was evaluated each cycle, organ response (OR) every 4th cycle (or end of treatment, if < 4 cycles given).
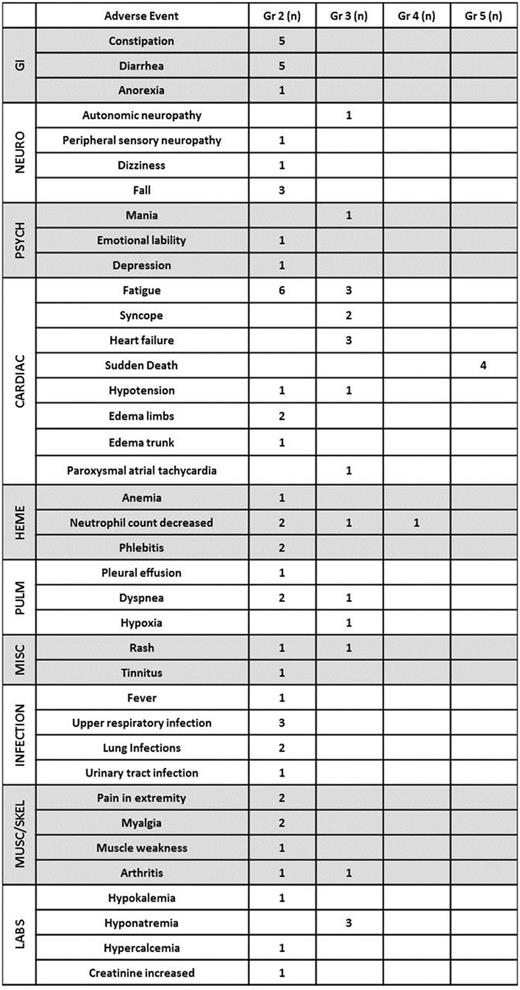
Results: Eighteen pts were enrolled: 17 AL, 1 LCDD. Median age 63.5 (range 49-76), Mayo original cardiac stage 1/2/3/unknown: 4/8/3/3. Enrollment into cohort 1/2/3/4/2b: 3/3/7/0/5. DLT was observed in cohorts 3 and 2b. The MTD was POM 3 mg/BTZ 1.0 mg/m2/DEX 20 mg (as all enrolled pts met criteria for baseline DEX dose reduction). The most common adverse events (AEs) of any severity were fatigue (n=15), diarrhea (n=8), constipation (n=8), anemia (n=12), and neutropenia (n=7). Table 1 summarizes Grade 2+ AEs. A median of 3 cycles was given (range 1-27). Eight pts have died, 4 of sudden death attributed to underlying cardiac AL while on protocol therapy. These four deaths (3 in pts with Mayo cardiac stage 2 disease, 1 with stage 3) occurred during therapy cycles 2, 2, 3, and 4. The HR rate was 50% (4 partial responses (PR), 3 very good partial responses (VGPR), 2 complete responses). Two of the 4 pts who died during therapy had had a HR (1 PR, 1 VGPR). Two pts had cardiac responses per consensus criteria (Gertz MA, et al, Am J Hematol 2005). Updated OR and OS data will be provided at the meeting. Two pts who failed to achieve ≥ PR with POM/BTZ/DEX subsequently responded after cyclophosphamide was substituted for POM. Four pts went on to autologous stem cell transplant (2 after VGPR, 2 after < PR) without issues mobilizing stem cells or unusual ASCT-related toxicity.
Discussion: POM/BTZ/DEX, while having light chain-lowering activity as frontline therapy for AL, was difficult to administer. DLTs were observed in 2 different treatment cohorts using doses of POM and/or BTZ and DEX below those typically used in frontline MM therapy. The spectrum of AEs was otherwise typical for these agents. The on-trial deaths, all attributed to cardiac AL, underscore the complexity of clinical research in pts with previously untreated AL. On-trial deaths were less common in POM/DEX trials for relapsed AL (Sanchorawala V, et al. Blood 2016; Palladini G, et al. Blood 2017). Thus, though the observed HR rate was similar to that for CyBorD (Palladini G, et al. Blood 2015), there are no current plans for a Phase 2 trial of POM/BTZ/DEX as frontline therapy for AL amyloidosis.
Zonder: Celgene: Consultancy, Research Funding; BMS: Consultancy, Research Funding; Prothena: Consultancy; Takeda: Consultancy; Pharmacyclics: Other: Data Safety Monitoring Committee; Janssen: Consultancy. Kukreti: Celgene: Honoraria; Amgen: Honoraria. Burt: Celgene: Speakers Bureau. Pregja: Takeda: Consultancy, Honoraria; Pharmacyclics: Other: Data Safety Monitoring Committee ; Celgene: Consultancy, Research Funding; BMS: Consultancy, Research Funding; Prothena: Consultancy; Janssen: Consultancy; Seattle Genetics: Consultancy, Honoraria. Matous: Celgene: Speakers Bureau; Multiple Myeloma Advisory Committee: Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal